A team from Forschungszentrum Jülich and RWTH Aachen University has developed a novel approach for a catalyst that combines the advantages of the two most commonly used catalysis methods. The new catalyst approach is based on the metal iridium and achieved five times the activity of previous reference systems in the lab – while maintaining high stability over several days. It had previously been considered difficult to achieve both high activity and long-term stability at the same time. These findings could help to further increase the efficiency of the highly active but expensive catalyst material iridium, thus making a significant contribution to advancing the use of green hydrogen as a climate-friendly energy storage solution. The team published its results in the renowned journal EES Catalysis of the Royal Society of Chemistry.
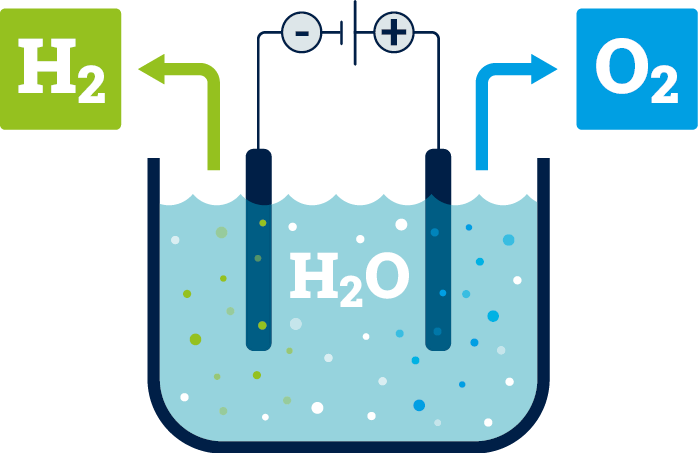
Green hydrogen plays an important role as an energy storage medium in the climate-friendly energy system of the future. In order to be used, it must be stored efficiently, transported and released again when needed. A key challenge here is to make the highly volatile gas as easy to use as possible. Carrier substances such as ammonia, methanol, formic acid and related molecules are possible options. Catalysts are needed to bind the hydrogen to these molecules and release it again. They accelerate the necessary reactions or make them possible or economically attractive in the first place.
Prof. Regina Palkovits. Photo: Research Centre/Jansen
Homogeneous and heterogeneous catalysis: a synthesis of two principles
The novelty of the Jülich-Aachen approach lies in the combination of two worlds of catalysis: homogeneous and heterogeneous catalysis. Homogeneous catalysis occurs when the catalyst and the reacting element, known as the reactant, are present in the same phase. For example, both are gaseous or liquid.
In heterogeneous catalysis, the catalyst is a solid and the element reacting with it is gaseous or liquid. The advantages of heterogeneous catalysis are that the catalyst and reactant can be separated cleanly and easily. This reduces costs. Homogeneous catalysis, on the other hand, has the potential to be more active and selective because all atoms of the catalyst material can be active. In addition, the catalyst can be precisely designed – its structure and chemical environment can be tailored specifically for a particular reaction. In a solid, however, the atoms within the nanoparticle are inactive because they do not come into contact with the reactants.
Normally, the team from the Catalyst Materials Division (INW-2) at the Jülich Institute for Sustainable Hydrogen Economy and the Aachen Chair of Heterogeneous Catalysis and Technical Chemistry focuses on heterogeneous catalysis. ‘With the new catalyst, we have tried to take the best from the other world, i.e. homogeneous catalysis, and use it for our world,’ explains Prof. Regina Palkovits, who heads the Aachen chair and the Jülich institute.
Very active and easily separable
Terpyridine plays a central role in this process – a molecule that binds metal atoms such as iridium firmly to itself. For the researchers from Jülich and Aachen, it was crucial to integrate the terpyridine structure, which can bind iridium very stably, into a polymer. A polymer is a chemical compound consisting of many small, repeating building blocks. The result is a so-called solid molecular catalyst (SMC), i.e. a solid, molecularly defined catalyst. ‘This allows the iridium to be separated from the reactant as in heterogeneous catalysis, in this case as a component of the terpyridine polymer,’ explains Keanu Birkelbach, first author of the publication. ‘At the same time, each iridium atom in the SMC forms a catalytically active centre, as is the case in homogeneous catalysis.’ This combination of higher activity and better separability is new. The iridium can be used more efficiently and also recycled. Given the high world market price, this offers great potential for savings. Iridium is currently around 50 percent more expensive than gold.
Next steps: scaling up and material alternatives
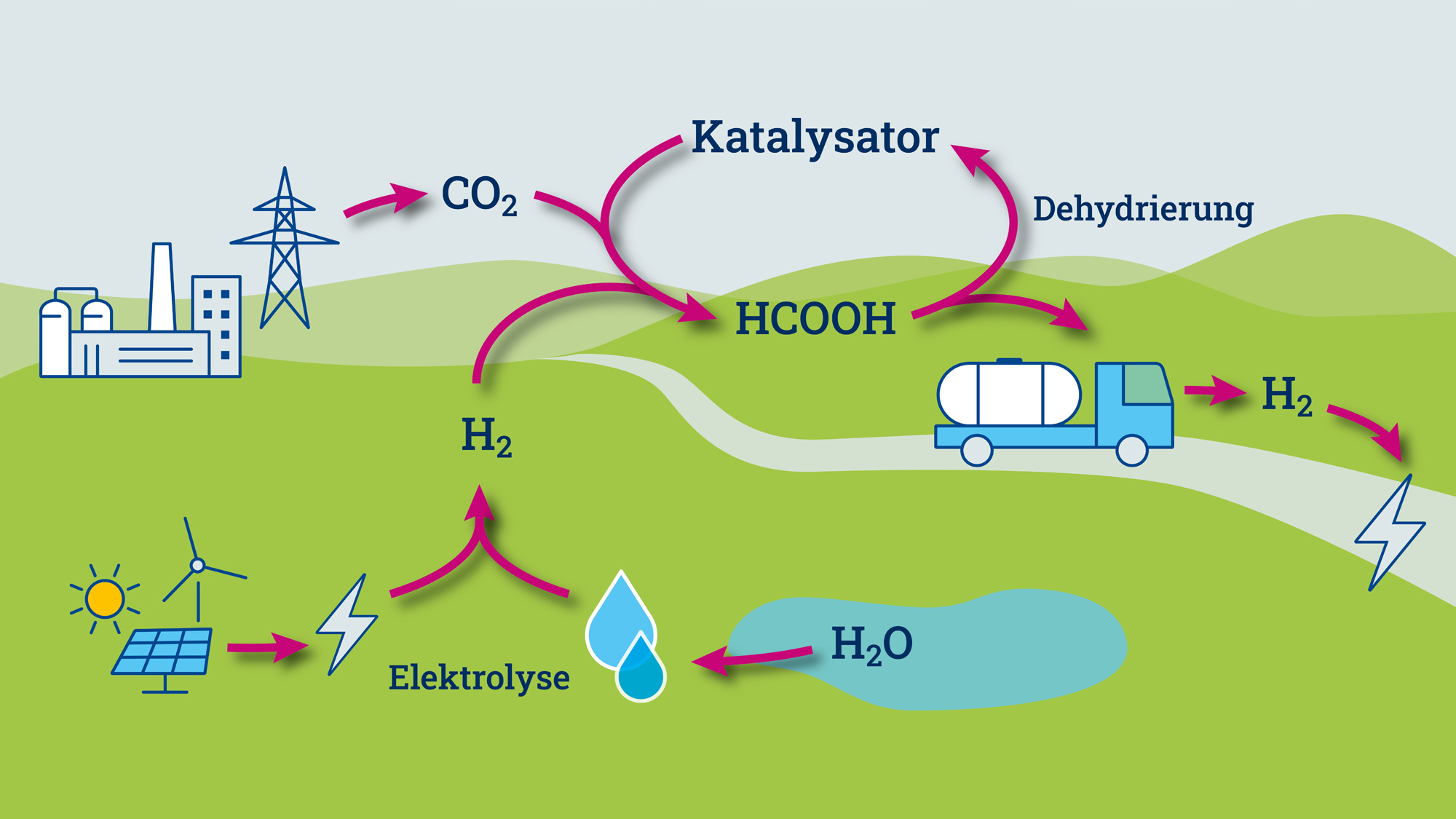
According to Keanu Birkelbach, further steps could include scaling up the reactor beyond the laboratory scale and replacing the expensive iridium with a cheaper, catalytically active metal. In addition, further hydrogen carrier molecules could be tested. In the laboratory, the team from Jülich and Aachen used the molecularly defined iridium catalyst to release hydrogen from formic acid.
Keanu Bikelbach. Photo: Jülich Research Centre/Limbach
The copyright for the images used on this website is held by Forschungszentrum Jülich, aligator kommunikation GmbH and
stock.adobe.com.